Uncategorized
Paxlovid: 3 Things to Know About Pfizer’s Promising COVID-19 Pill
Following Merck’s announcement of its COVID-19 pill Molnupiravir, Pfizer announced it was also applying for authorization of Paxlovid, their own COVID-19 pill.
After coming up with its COVID-19 vaccine, pharmaceutical company Pfizer announced it was rolling out its own anti-viral drug. The company announced it has given permission to generic manufacturers to produce Paxlovid. The new anti-viral drug comes following the announcement of Merck’s Molnupiravir. It’s unclear yet when it will be available. But here are some details we were able to find out about the new Paxlovid, the new COVID-19 pill.
Latest news about Paxlovid!
As Omicron cases climb, the Philippine FDA has decided to grant a special permit for the generic version of Paxlovid which is currently nicknamed — Bexovid. The said generic form has the chemical combination of two anti-virals and is something that should be taken twice for 5 days. “It was examined and researched by Pfizer in the USA — they found in their data that on all the patients who were given it, it reduced their hospitalization and death to about 90 percent,” explains DOH Undersecretary Leopold Vega during a press interview last January 11, 2022.
Permission for Generic Manufacturing

Pfizer said that it was allowing generic manufacturers to produce the experimental anti-viral drug to 95 low and middle-income countries through a partnership with the international group Medicines Patent Pool (MPP).
Initial Clinical Trial Results
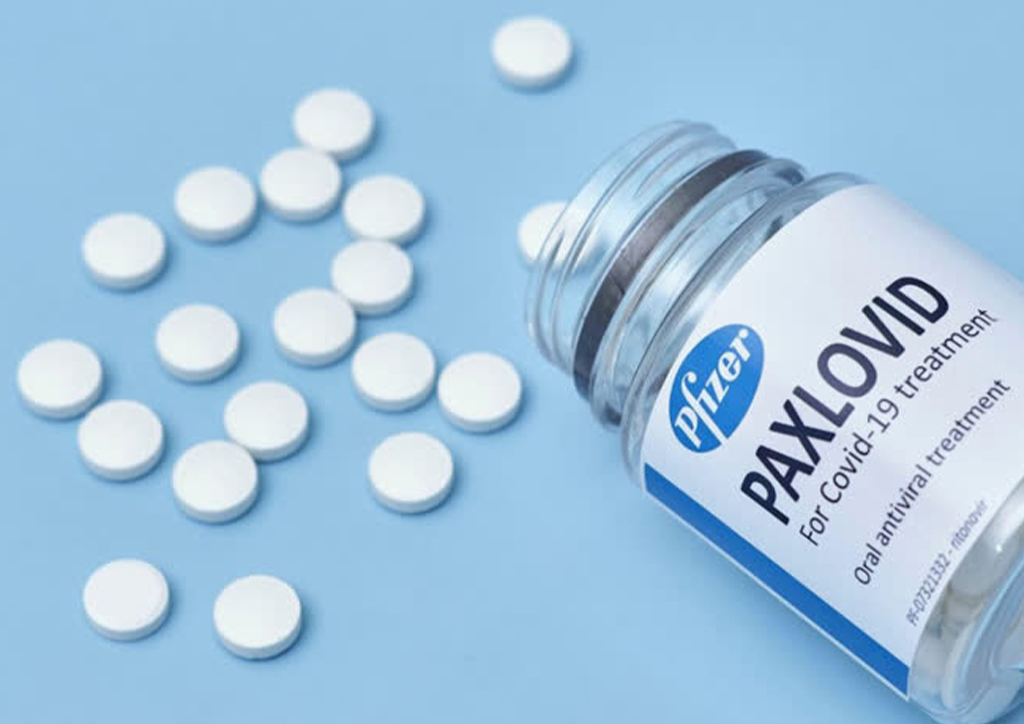
Pfizer shared that the pill has cut the high chances of hospitalization or death of adults with severe disease at 89%. This means that the drug was able to help reduce the condition of those affected by mild signs of COVID-19 before it even got worse.
Another COVID-19 Pill Coming Soon

According to Pfizer, they are expected to manufacture at around 180,000 treatment courses by the end of the year and the rest in 2022. The company has applied for emergency authorization of the drug in the US.
On Thursday, November 18, the company announced it has sealed a $5.29 billion deal with the US government to deliver 10 million courses of the anti-viral drug.
There’s no specific time frame on when the drug will be available in other countries.
Feeling Hopeful
With vaccines now available and the arrival of anti-viral pills, people have a better chance of fighting COVID-19. But even with the promising treatment, moms and dads should still remember that the disease is still out there. We still need to follow health protocols to save lives and prevent kids, especially aged under 12, from getting the virus.
Updating yourself with health news? Here are some stories:
UPDATED: Would You Let Your Child Get COVID-19 Pfizer Vaccine? Here’s What You Need To Know
10 Tips for Parents to Get Proper Sleep
Is Molnupiravir COVID-19’s Worst Nightmare?